|
Many chemical elements are present in nature as a mixture of different stable isotopes with different atomic weight.The isotopic composition of a compound may change depending on the conditions in which it was formed and the physico-chemical reactions in which it intervenes. This is because heavier isotopes tend to be discriminated during that reactions, resulting in a fractionation of different isotopes between the products of the reaction and the remaining substrate. For instance, lighter molecules of water (that is, those containing lighter isotopes of H and O) tend to evaporate faster than the heavier ones. As a result, water vapour results enriched in light molecules, while liquid water keeps the heavy ones. |
source: http://www.co2web.info/ |
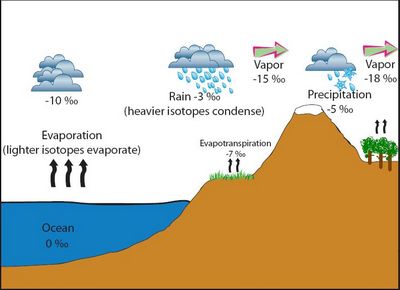
A schematic diagram of the isotope fractionation process via evaporation, condensation, and evapotranspiration (source http://serc.carleton.edu/microbelife/research_methods/environ_sampling/stableisotopes.html)
We have added the measurement of both stable and radio-isotopes to the routinary monitoring scheme in the Aigüestortes i Estany de Sant Maurici National Park, with the aims of:
1) Comparing the isotopic composition of water and several solutes in atmospheric deposition in the bottom and the highest areas of the Sant Nicolau valley.
2) Carrying out solute mass-balances in three lakes at different altitude, to assess changes in the isotopic composition and infere which are the dominant biogeochemical processes within the lakes.
3) Examinating the fluxes of isotopes to and from the sediments in two lakes, using sediment traps and monitoring changes in the deepest zones of the lakes. Thermistor chains are installed in the lakes to monitor in detail stratification and mixing of the water column.
4) Measure the inputs of fine and coarse suspended matter in two lakes, and determine its isotopic composition. For this, drift traps are installed at the inlet and outlet of the lakes.
5) Modeling the transfer of radio-isotopes from the atmosphere to soil and snow cover, and from these to the lakes.
The isotopic composition of meteoric water (that is, its 2H and 18O content) can be used as a tracer of the pathway it follows within a catchment, and the effect of evaporation during its flowing down the slopes and the valley.
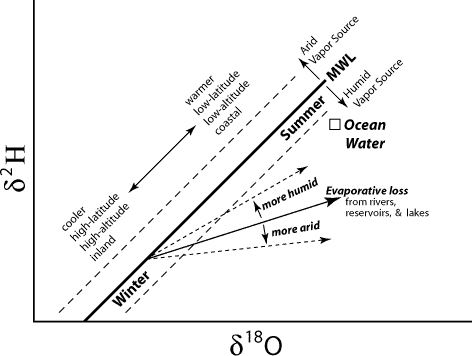 |
| 2H and 18O in meteoric waters fall along a line reflecting the differences in their equilibrium fractionation factors (MWL, thick line). The diagram summarizes how hydrologic processes affect the isotopic composition of water causing deviations from the MWL (source: http://web.sahra.arizona.edu/programs/isotopes/oxygen.html#4) |
|
Radioactive isotopes, of either natural or artificial origin, can be used as tracers of numerous environmental processes according to their half-life, source and specific biogeochemical characteristics. From July 2013 to July 2014 samples of precipitation (20), snow cover (8), ice cover (12), water column (24) and soil (40) were collected in the Sant Nicolau valley within the ISÓTOPOS project. The aim is to estimate the transfer of the natural isotopes 7Be, 210Pb and 210Po between the atmosphere - snow/ice cover - lakes. This shall allow evaluating the efficiency with which the snow/ice cover intercepts the atmospheric fluxes of species of interest in the system, such as stable isotopes, and studying their fate when melting occurs. The analysis of 7Be and 210Po/210Pb are being conducted at the Laboratori de Radioactivitat Ambiental (Universitat Autònoma Barcelona).
Sampling radioisotopes in Lake Llebreta water column |
|
Sampling radioisotopes in the snowcover |