The rationale for the ISOTOPOS project
|
Many chemical elements are present in nature as a mixture of different stable isotopes with different atomic weight.The isotopic composition of a compound may change depending on the conditions in which it was formed and the physico-chemical reactions in which it intervenes. This is because heavier isotopes tend to be discriminated during that reactions, resulting in a fractionation of different isotopes between the products of the reaction and the remaining substrate. For instance, lighter molecules of water (that is, those containing lighter isotopes of H and O) tend to evaporate faster than the heavier ones. As a result, water vapour results enriched in light molecules, while liquid water keeps the heavy ones. |
source: http://www.co2web.info/ |
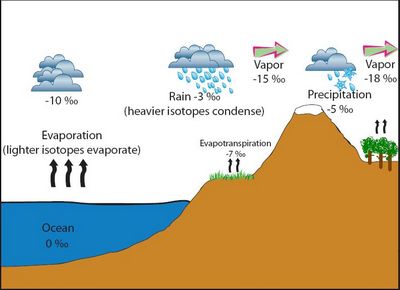
A schematic diagram of the isotope fractionation process via evaporation, condensation, and evapotranspiration (source http://serc.carleton.edu/microbelife/research_methods/environ_sampling/stableisotopes.html)
Some elements with different stable isotopes are part of living matter. For this reason, the isotopic analysis has an application to ecological and biogeochemical studies. The isotopic composition of elements in water, nutrients, metabolites and organic matter (both living and dead) offers a valuable information on their origin and the processes to which they have been submitted. The main applications of isotopic analysis in ecology are the identification of the sources of substrates participating in the biogeochemical reactions, and the quantification of the relative importance of each of these reactions in the functioning of the ecosystem.
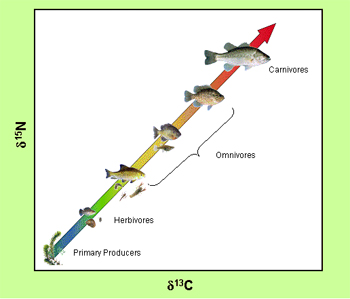
An example of the use of stable isotopes in ecological studies: change of the C and N isotopic composition of tissue from biota along the food web in an aquatic ecosystem (source: http://wwwrcamnl.wr.usgs.gov/isoig/projects/fingernails/foodweb/isotopes.html)
However, the isotopic signature of substrates may change during the seasonal cycle of an ecosystem, and on the long term too. Also, many products of a reaction are on its turn substrates for another reaction. Therefore, a good knowledge of how the isotopic composition changes in the sources and which reactions are predominant at each given time is needed in order to interpret correctly the observed isotopic composition. In this project we aim to include the isotopic analyses of several compounds of interest into the on-going monitoring program of the biogeochemistry of surface waters in the Aigüestortes i Estany de Sant Maurici National Park, with the aim of quantifying the range and mode of variation of the isotopic signature of substrates and identifying the main biogeochemical reactions that cause these changes.
Isotopic analysis has been used in ecological research since several decades. However, it has not been of general use until recently. It requires sophisticated instrumentation and highly skilled operators. The preparation of samples for analysis was time consuming, and the available analytical techniques allowed the analysis of only a few samples a day. The resulting high cost per analysis prevented an extended use. Nowadays, the development and automation of techniques and instruments has lowered prices. Specialised facilities offer now affordable services. Isotopic analyses are a standard technique widely used, and its routinary use in long term monitoring programs is feasible.

Isotope-ratio mass spectrometer used to measure stable isotope ratios, with gas bench in foreground (source: http://en.wikipedia.org/wiki/Isotope-ratio_mass_spectrometry#Gas_source_.28.27stable_isotope.27.29_mass_spectrometry)
In addition to stable isotopes, we want to assess the potential of some radioisotopes as a monitoring tool. Radioisotopes, both natural and artificial, are good tracers of environmental processes at varying time scales. In particular, they can serve to trace atmospheric inputs into soils and the snow and ice cover of a catchment and see what is the role in regulating the fluxes of those airborne inputs to streams and lakes. Thus, the efficiency of interception and the dynamics of transfer during snow melting can be quantified, and this quantification can be translated to other chemicals, like nutrients, pollutants and, as in the case in this project, stable isotopes.
The project “ISOTOPOS: Pilot study for the use of stable and radiactive isotopes in the monitoring and long term investigations (LTER) on the aquatic ecosystems in National Parks” is funded by the Organismo Autónomo de Parques Nacionales - MAGRAMA through grant #702/2012 within its Research Program in National Parks (http://www.magrama.gob.es/es/parques-nacionales-oapn/programa-investigacion/)